DENR controls JAK2 translation to induce PD-L1 expression for tumor immune evasion
Baiwen Chen#, Jiajia Hu#*, Xianting Hu#, Huifang Chen, Rujuan Bao, Yatao Zhou, Youqiong Ye, Meixiao Zhan, Wei Cai*, Huabin Li*, Hua-Bing Li*. Nature communications. 2022 April 19
PD1/PD-L1 immunotherapy has demonstrated remarkable efficacy in the treatment of various cancers and ushered in a new era of tumor immunotherapy; however, there are still problems such as poor therapeutic effects and even resistance in patients. Research into the molecular mechanism of PD-L1 regulation is urgently needed. In particular, it is unclear whether there is a specific RNA-binding protein that regulates PD-L1 expression in tumor cells to mediate tumor immune escape.
In this study, our team performed a customized CRISPR/Cas9 Screening focused on 1467 novel RNA binding proteins, and found that knockdown of the density-regulated translation re-initiation factor DENR significantly reduced PD-L1 expression in multiple cell lines. In vitro, DENR-depleted tumor cells demonstrated a higher percentage of apoptosis when co-cultured with CD8+ T cells. In vivo, tumor lacking DENR possessed smaller tumor volume with enhanced CD8+ T cell infiltration.
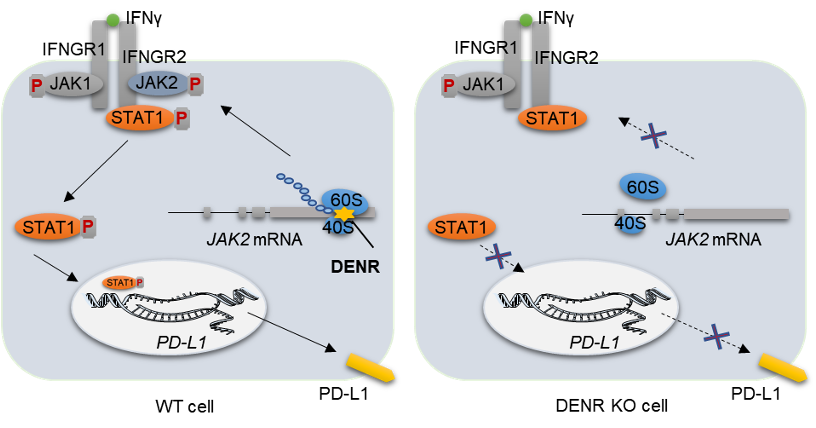
Clinical data analysis showed that melanoma patients with low DENR expression were more sensitive to anti-PD1 treatment. As a consequence of DENR depletion, PD-L1 mRNA levels in tumor cells were significantly lowered, along with a diminished IFNγ-JAK-STAT signaling pathway. Interestingly, JAK2 protein was significantly reduced, but JAK2 mRNA was not affected. DNR depletion caused an accumulation of ribosomes on the uORF sequence in the 5'UTR of JAK2, reducing JAK2 protein translation.
To summarize, DENR enhances JAK2 protein translation efficiency, which in turn boosts PD-L1 expression via the JAK2-STAT1 signaling pathway, thus facilitating tumor immune escape. This research proposed a unique strategy to improve immunotherapy efficacy by targeting DENR.